Brain Modeling Tools: BMTK, SONATA, VND
Type: Software,
Keywords: Neural network processing, Multiscale modeling, Reproducibility and open science, Neuronal model visualization, Simulation, Computational neuroscience
Software tools for building, simulating, sharing, and visualizing neuronal network models
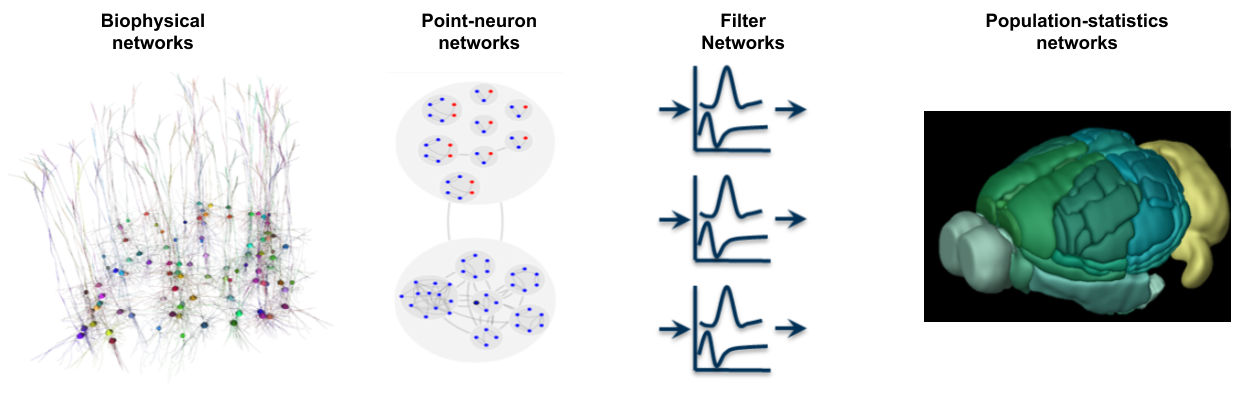
The Brain Modeling Toolkit (BMTK), SONATA, and Visual Neuronal Dynamics (VND) are mutually integrated software tools that are particularly suited to support large-scale bio-realistic brain modeling, but are applicable to a variety of neuronal modeling applications. BMTK is a suite for building and simulating network models at multiple levels of resolution, from biophysically-detailed, to point-neuron, to population-statistics approaches. The modular design of BMTK allows users to easily work across different scales of resolution and different simulation engines using the same code interface. The model architecture and parameters, as well as simulation configuration, input, and output are stored together in the SONATA data format. Models and their output activity can then be visualized with the powerful rendering capabilities of VND.
* BMTK: Software package with consistent Python interface to build, run, and analyze neuronal network models at a range of resolutions using NEURON, NEST, filter networks, or diPDE.
* BMTK: Allows specification of realistic morphologies, custom network inputs, biophysical properties, and connection probabilities.
* BMTK: Parallelization and efficiency for large-scale models.
* SONATA: Open data format for storing model network, simulation details, inputs, and model outputs; shareable, convertible, supported in numerous simulation environments and APIs.
* VND: Interactive and high-performance rendering of network models for displaying, analyzing, and animating network morphology, connectivity, and activity.
*Building network models at multiple levels of resolution, such as using the biophysically-detailed, point-neuron, or population-statistics approximations for network nodes.
*Incorporating a broad range of diverse cell types, connectivity patterns, and biological mechanisms in the model networks, facilitating highly realistic, data-driven modeling.
*Carrying out simulations of large and heterogeneous networks in a scalable and efficient way, using single CPUs, clusters, or supercomputers.
*Simulating network responses to diverse stimuli and various perturbations, dissecting mechanistic contributions.
*Recording simulated neural activity and various signals, such as the extracellular electric potentials.
*Comparing in silico predictions to matched experimental observations.
*Integration of diverse data into a bio-realistic model of mouse cortical area V1, containing 230,000 neurons of 17 different types, realistic connectivity and visual inputs, and reproducing many aspects of experimentally observed visual physiology.
*Billeh, Y. et al. Systematic Integration of Structural and Functional Data into Multi-scale Models of Mouse Primary Visual Cortex. Neuron. 106 (3):388-403 (2020).
*The V1 model in SONATA format was used to investigate the cortical circuit mechanisms underlying behavioral performance and physiological responses in a change-detection task.
*Scherr, F. and Maass, W. Analysis of the computational strategy of a detailed laminar cortical microcircuit model for solving the image-change-detection task. bioRxiv 2021.11.17.469025 (2021).
*The V1 model was used to study the cell type and circuit mechanisms, determining generation of the local field potential in the cortex.
*Rimehaug, A.E. et al. Uncovering circuit mechanisms of current sinks and sources with biophysical simulations of primary visual cortex. bioRxiv 2022.02.22.481540 (2022).
*Learn one accessible Python interface to access multiple simulators at different levels of resolution.
*Convert between different scales of resolution.
*Use built-in or custom stimuli or spike train inputs.
*Integrate multiple types of data into models (morphological, connection strength and probability, biophysical properties).
*Use with high-performance computing.
*Standardize and share models with the SONATA file format.
*Computationally efficient storage of model details in the SONATA format.
*Visualize neuron and network models with selection and 3D interactive graphics.
*Make images and movies of models and of simulated network activity.
*Large-scale bio-realistic models may require substantial computing resources and are dependent on availability of detailed data for parameters.
*VND is currently under development, with more functionality being added. BMTK will continue to add user requested features and modifications.
*Python 2.7+ or Python 3.6+ and associated libraries.
*Simulation engine used in conjunction with, e.g., NEURON simulator v7.4 and above or NEST simulator v2.11 and above.
*See: https://alleninstitute.github.io/bmtk/installation.html.
*Dai et al. The SONATA data format for efficient description of large-scale network models. PLoS Comput Biol. 16(2):e1007696 (2020).
*Dai et al. Brain Modeling ToolKit: An open source software suite for multiscale modeling of brain circuits. PLoS Comput Biol. 16(11): :e1008386 (2020).
*Optional registration: https://secure2.convio.net/allins/site/SPageServer/?pagename=modeling_tools
*Github: https://github.com/AllenInstitute/bmtk
*Allen Brain Map forum: https://community.brain-map.org/
*Allen Brain Map portal: https://portal.brain-map.org/explore/models
*On Neuroscience Gateway: https://nsgprod.sdsc.edu:8443/portal2/tools.action
*BMTK Documentation: https://alleninstitute.github.io/bmtk/bmtk/bmtk.html
*VND Documentation: https://www.ks.uiuc.edu/Research/vnd/current/docs.html
*Modeling software workshop: https://alleninstitute.org/what-we-do/brain-science/events-training/2022-modeling-workshop/
*Show us your BRAINs 2021 winner: https://braininitiative.nih.gov/sites/default/files/pdfs/model_of_mouse_v1_barry_isralewitz_braincontest2021.png
Anton Arkhipov, Associate Investigator
Allen Institute
TEAM / COLLABORATOR(S)
Anton Arkhipov, Associate Investigator, Allen Institute
Barry Isralewitz, Postdoctoral Research Associate, University of Illinois at Urbana-Champaign
Emad Tajkhorshid, Professor, University of Illinois at Urbana-Champaign
John Stone, Senior Research Programmer, University of Illinois at Urbana-Champaign
Kael Dai, Software Engineer III, Allen Institute
Mariano Spivak, Postdoctoral Researcher, University of Illinois at Urbana-Champaign
Xiao-Ping Liu, Software Engineer I, Allen Institute
FUNDING SOURCE(S)
NIH U24NS124001

