Myomatrix Arrays
Type: Electrophysiology / Probes,
Keywords: Flexible multi-electrode array (MEA) for high resolution EMG, Electromyography electrodes, 3D MEA device, Electromyogram (EMG) recording, Neurophysiology, Motor system, Vertebrate and Invertebrate animals
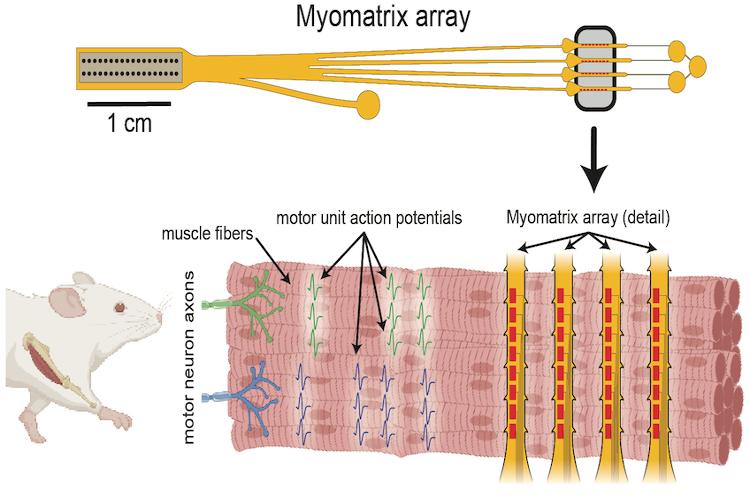
Micro-scale EMG arrays for recording single- and multi-unit activity
Our microfabrication process yields flexible high-density electrode arrays (called Myomatrix arrays) with low impedance enabling researchers to record muscle activity with resolution as high as single motor units in freely behaving animals and from a range of species including mice, rats, songbirds, and non-human primates. The arrays come in a variety of configurations designed for use in acute and chronic recording paradigms.
*By developing advanced nanofabrication tools for manufacturing flexible, multi-electrode arrays, we are creating a new class of electromyography (EMG) electrodes capable of recording large populations of single-unit recordings from muscle fibers during behavior.
*Devices have been used to record EMG signals in humans, nonhuman primates, rats, mice, songbirds, frogs, fish and insects.
*Array fabrication results in high signal-to-noise (SNR) in vivo electromyogram (EMG) recordings.
*The flexible arrays can be targeted to one or more muscles and yield high-resolution recordings of motor activity during free behavior.
*The arrays come in a variety of configurations designed for use in acute and chronic recording paradigms.
These flexible, high-density electrode devices (“Myomatrix arrays”) can record muscle activity (EMG) at high resolution, in freely moving animals, and across a wide range of muscles and species.
The multi-threaded arrays are capable of recording from multiple muscles at once, allowing for evaluation of coordination across muscles in a single animal.
The flexible polymer used in the fabrication allows for high-resolution EMG across a range of muscle contraction and behaviors.
Flexible high-resolution EMG arrays capable of high SNR recordings from the muscles of multiple species:
*High-quality EMG recordings were acquired using 32-channel Myomatrix electrode simultaneously during quiet respiration in eight male Bengalese finches.
*Quantitative behavioral analysis of mouse locomotion and single motor unit recordings in triceps using Myomatrix arrays were combined to investigate how the nervous system controls locomotor speed.
*To confirm a biomechanical model of reaching task in mouse, the EMG activity of a subset of forelimb muscles was recorded and used to assess the accuracy of the muscle patterning predicted by the model.
*Myomatrix arrays yielded single motor unit recordings during cued reaching movements in non-human primates.
*Human
*Non-human primate
*Rat
*Mouse
*Songbird
*Frog
*Zebrafish
*Insects
*Myomatrix arrays generate high SNR measurements over a longer duration of time which is important for detecting and analyzing smaller units which are otherwise lost in noise.
*The arrays come in a variety of designs which provide options for high-resolution EMG recording across one or multiple muscles in a single recording session or for recording from small or difficult to surgically access muscles.
*With better signal fidelity, individual units can be identified more reliably and for longer periods of time, which will allow more advanced analysis techniques that can be used to understand how nervous systems control behavior.
*Myomatrix arrays provide EMG recordings that are resistant to movement artifacts and stable over time in both acute and chronic preparations.
*Combining Myomatrix recordings with high-density brain recordings or targeted manipulations of neural activity can reveal how central circuits shape and reshape motor activity and reveal how neural dynamics in cortical, subcortical, and spinal circuits shape the spiking patterns of individual motor neurons.
*Designs for non-traditional research animals are still in the prototyping phase.
*Data acquisition requires systems that connect to a 36-pin Omnetics connector.
The arrays electrically connect to data acquisition systems using a 36-pin Omnetics connector. Thus, one must use a system compatible with this particular connector or build an adapter to connect the array to your personal data acquisition system.
A typical set-up for recording in vertebrate species includes the Myomatrix array connecting to an Intan RHD bi-polar headstage via the 36-pin Omnetics plug. The headstage connects to an Intan or Open Ephys data acquisition board using an SPI cable and a USB cable connects the acquisition board to a computer to run the acquisition software.
*K Thomas et al., (2024) Motor unit mechanisms of speed control inmouse locomotion. bioRxiv 2024.12.29.628022; https://doi.org/10.1101/2024.12.29.628022
*L Qin et al., (2024) Separation of fascicles for motor unit separability in reinnervated muscles for neuroprosthesis application. 46th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 2024, pp. 1-4, doi: 10.1109/EMBC53108.2024.10782049
*JM Barrett et al., (2024) Hand–Jaw Coordination as Mice Handle Food Is Organized around Intrinsic Structure–Function Relationships. J Neurosci 16 Oct., 44 (42) e0856242024; https://doi.org/10.1523/JNEUROSCI.0856-24.2024
*JI Gilmer et al., (2024) A novel biomechanical model of the mouse forelimb predicts muscle activity in optimal control simulations of reaching movements. BioRxiv preprint. DOI: https://doi.org/10.1101/2024.09.05.611289
*PM Anschutz et al., (2024) Flexible EMG arrays with integrated electronics for scalable electrode density. BioRxiv preprint. DOI: https://doi.org/10.1101/2024.07.02.601782
*EA Kirk et al., (2024) An output-null signature of inertial load in motor cortex. Nat. Comms. 15, 7309. DOI: https://www.nature.com/articles/s41467-024-51750-7
*J Lu et al., (2024) Opto-Myomatrix: μLED integrated microelectrode arrays for optogenetic activation and electrical recording in muscle tissue. BioRxiv preprint DOI: https://doi.org/10.1101/2024.07.01.601601
*JJ Kim et al., (2024) Myo-optogenetics: optogenetic stimulation and electrical recording in skeletal muscles. bioRxiv 2024.06.21.600113; doi:https://doi.org/10.1101/2024.06.21.600113
*EA Kirk and BA Sauerbrei. (2024) Electromyography: Accessing populations of motor units (Commentary on Chung et al (2023)) ELife 2024;Jan 4:13:e94764. DOI: https://doi.org/10.7554/eLife.94764
*B Chung et al., (2023) Myomatrix arrays for high-definition muscle recording. ELife Dec 19:12:RP88551 DOI: 10.7554/eLife.88551.
*J Lu et al., (2022) High-performance flexible microelectrode array with PEDOT:PSS coated 3D microcones for electromyographic recording. Annu Int Conf IEEE Eng Med Biol Soc. Jul; 2022: 5111–5114. DOI: 10.1109/EMBC48229.2022.9871052
*M Zia et al., (2020) Flexible multielectrode arrays with 2D and 3D contacts for in vivo electromyography recording. IEEE Trans. Comp. Packag. Manufac. Tech. 10(2): 197-202. DOI: 10.1109/TCPMT.2019.2963556
EMUsort – a motor unit specific spike sorter at https://github.com/snel-repo/EMUsort
“Digital sutures”. International patent published by the World Intellectual Property Organization (WIPO) on March 9, 2023 as application 2023/034258A1.
Sam Sober, Associate Professor
Emory University
TEAM / COLLABORATOR(S)
Center for Advanced Motor BioEngineering and Research (CAMBER)
Muhannad Bakir, co-Director
Kristen Frenzel, Associate Director of Education and Outreach
Amanda Jacob, Associate Director for Operations and User Training
Sam Sober, Director
Taniel Winner, Associate Director of Translational Research
Muneeb Zia, Associate Director of Technology and Innovation
FUNDING SOURCE(S)
NIH U24NS126936
NIH R01NS109237

