Summary: As part of Women’s History Month, we highlight four inspiring female scientists and their exciting, innovative BRAIN-funded research.
In her laboratory at Columbia University, Dr. Elizabeth Hillman is busy making modifications to her new microscopy system. Called SCAPE (swept confocally aligned photon excitation), this platform she has spent several years developing can capture the firing of neurons and the flow of blood through neurovasculature in real time. It can even image living, moving organisms such as zebrafish, fruit fly larvae, or rats. With a grant from the BRAIN (Brain Research through Advancing Innovative Neurotechnologies) Initiative, she is now working to make this technology available to other neuroscientists, who can use it for discoveries of their own.
BRAIN builds collaborations
Scientific advancement is achieved by fostering partnerships and trying bold, new ideas. An interdisciplinary approach has brought about the greatest discoveries and applications in health and medicine, especially in the past few decades. Now, the BRAIN Initiative is similarly boosting neuroscience research by enabling collaborations and the development of novel technologies.
The BRAIN Initiative itself was formed from a collaboration between ten of the institutes and centers of the National Institutes of Health (NIH) and several other federal research agencies, including the National Science Foundation (NSF), United States Food and Drug Administration (FDA), Intelligence Advanced Research Projects Activity (IARPA), and Defense Advanced Research Projects Agency (DARPA). Soon thereafter, these federal partners teamed up with non-federal groups – the Allen Institute for Brain Science, The Kavli Foundation, the Simons Foundation, and Janelia/Howard Hughes Medical Institute – to form the BRAIN Initiative Alliance. The program supports scientists who wish to expand the scope of their work and test high-risk, high-reward ideas.
As a part of its objective, the BRAIN Initiative encourages diversity within the neuroscience arena. It provides great opportunities for new or early-stage investigators, enabling scientists from a variety of backgrounds to begin their careers or access advanced research technologies. More women than ever are leading neuroscience research teams and developing novel neurotechnologies, and many of them have been supported by BRAIN awards.
Elizabeth Hillman

Dr. Elizabeth Hillman grew up being very curious about how things work. She had a knack for putting things together, and a fascination with images of the inner workings of complex structures like the human body. Following degrees in physics, where she developed expertise in optics and laser technology, she spent time working in medical imaging where she says she got caught up in the “beautiful revolution of GFP (green fluorescent protein).” There, she witnessed how imaging technology could be applied to medical biology to better understand health and disease.
In neuroimaging, Hillman noticed several challenges. One was the difficulty of scanning brain tissue beyond a superficial level. Another was the imaging speed which was required to detect neuron signaling and interactions. Also, there needed to be a robust method for interpreting data obtained from brain scans in a meaningful way.
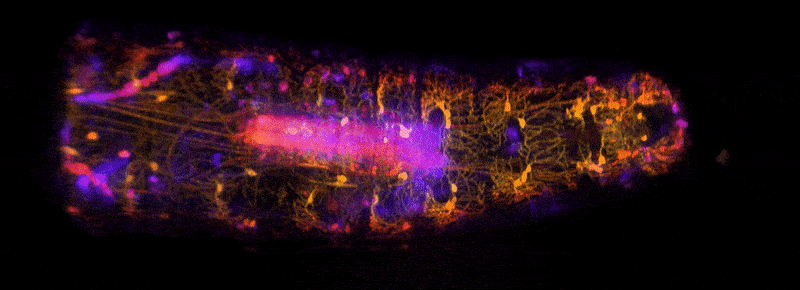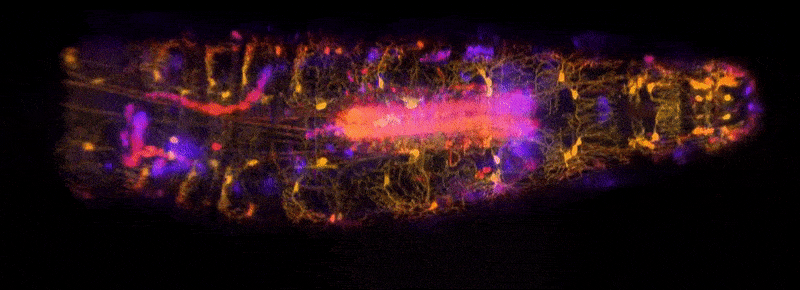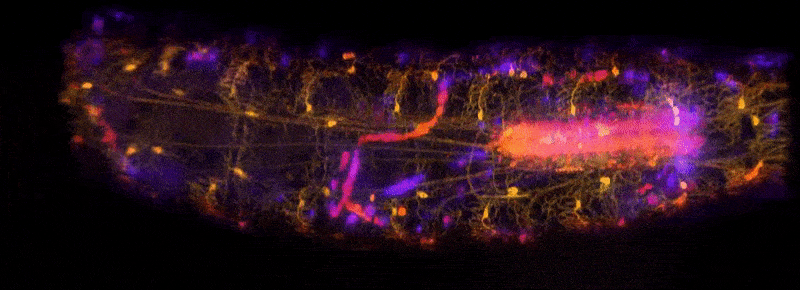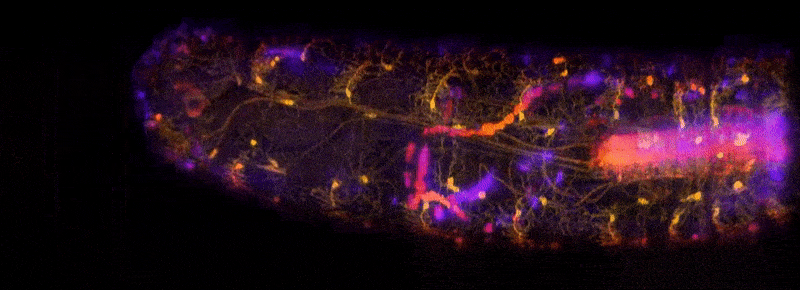
To address these challenges, Hillman brought her physics and engineering experience to her neuroscience lab at Columbia University, where she is a professor of biomedical engineering and of radiology. There, she and her team have been inventing microscope technologies to image brain structures and activities in three dimensions. “We didn’t really follow any kind of convention,” she says. So far, she has built nearly 12 different brain imaging systems. The SCAPE system can now acquire 3D data at speeds 10 to 100 times faster than other modern microscopy technologies with cellular-level resolution.
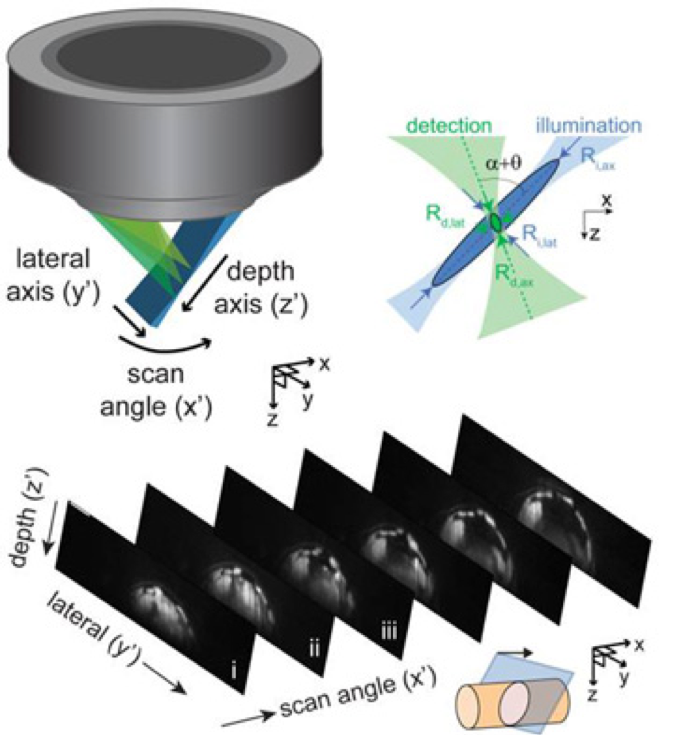
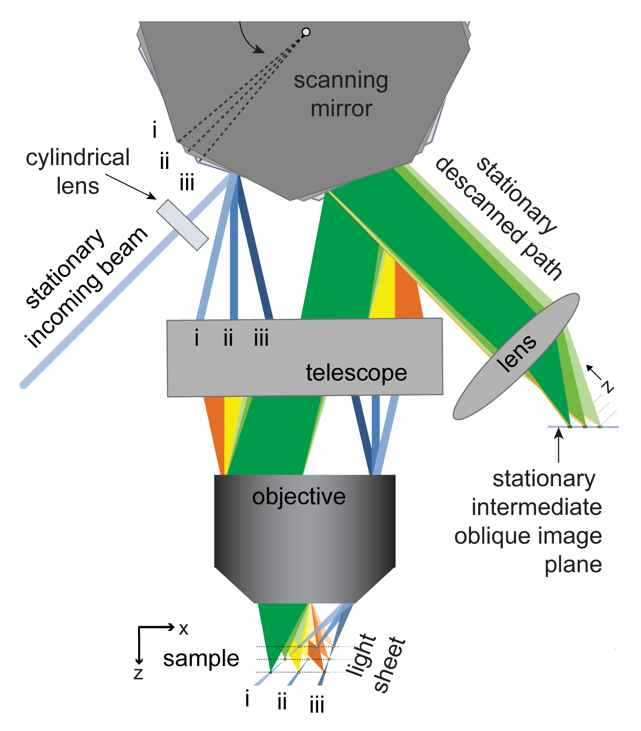
Because of the immense utility of this system, Hillman is working to help other laboratories obtain their own SCAPE microscopes and learn how to analyze data with them. Her BRAIN award has enabled her to create an effective team devoted to building SCAPE microscopes and introducing them to other researchers. Before, Hillman did not have much experience finding collaborators or putting together a support team. “BRAIN gave me that confidence and the resources for that,” she says. Hillman has been engaged in outreach as well, traveling to other institutions and conventions where she can demonstrate SCAPE’s capabilities.
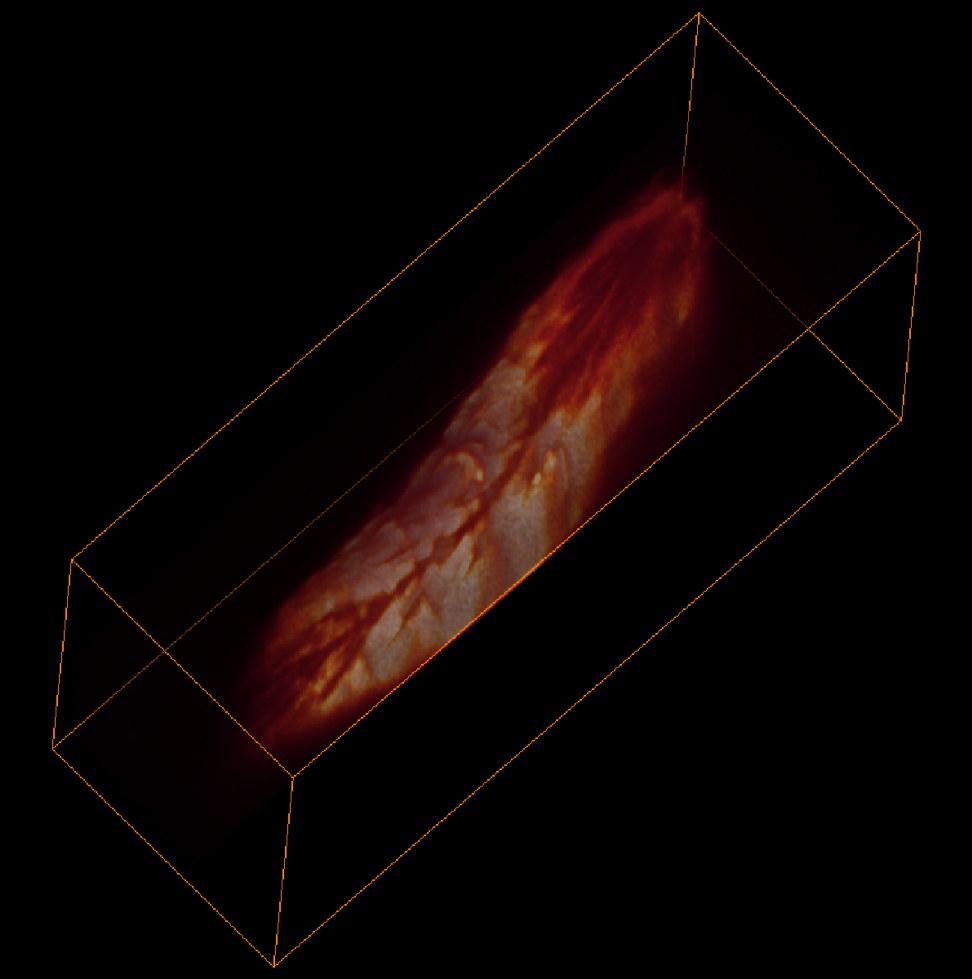
Image credits: Venkatakaushik Voleti, Elizabeth Hillman, Cesar Mendes.
Part of Hillman’s current focus is scaling up to meet the demand for fast, high-resolution neuroimaging instruments. She hopes to further develop the SCAPE system for a wider range of applications, such as medical uses on the human brain. In the meantime, she is also developing a support system for other SCAPE users, such as writing easy-to-follow instructions on how to build a SCAPE microscope and determining how best to maintain one.
Mala Murthy

Dr. Mala Murthy is uncovering the ways in which the brain processes sensory information. She is a professor of neuroscience and molecular biology at Princeton University, where she is the principal investigator of an acoustic communication laboratory. Learning how fruit flies (Drosophila melanogaster) use audible vibrations to communicate can give insight into the neurological mechanisms underlying speech recognition and communication disorders in humans.
The Murthy lab is working to understand the brain circuitry and neural connections involved in interpreting auditory signals and translating them into behaviors. Male fruit flies use wing vibrations to produce songs as they court females, which the females assess to select a mate. The goal of Murthy’s research is to understand the neural mechanisms of the processes of interpreting and responding to sounds.
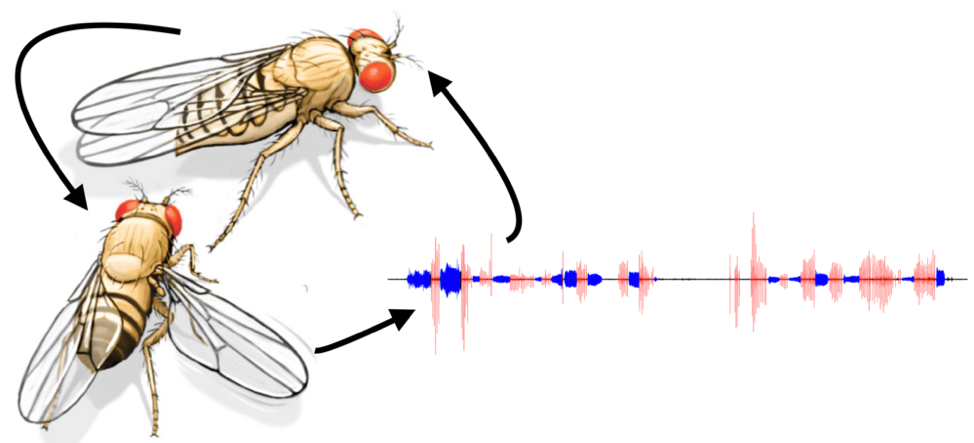
To help achieve this, Murthy collaborated with physics researchers to adapt state-of-the-art equipment and computational modeling techniques to assay the activities of neurons in the fruit fly brain during courtship. “This kind of work relies on new and evolving technology, the majority of which has been funded by the BRAIN Initiative,” Murthy says. “In fact, the dream of simultaneous recording of all neurons within the brain of a behaving animal seemed far-fetched 10 years ago, but it is now achievable within a number of model systems, including flies. These tools and technologies have been pushed forward, thanks to the BRAIN Initiative.”
The findings from Murthy’s work will have multiple applications to human health. Some of the neuropathological complications of diseases (e.g., autism spectrum disorder and Parkinson’s disease, as well as brain injury and stroke), involve a deficit in the brain’s pattern recognition and communication pathways. Ultimately, she hopes that her research will lead to the invention of simple neural prosthetic devices that could restore function to these pathways in cases of injury or disease.
Ute Hochgeschwender

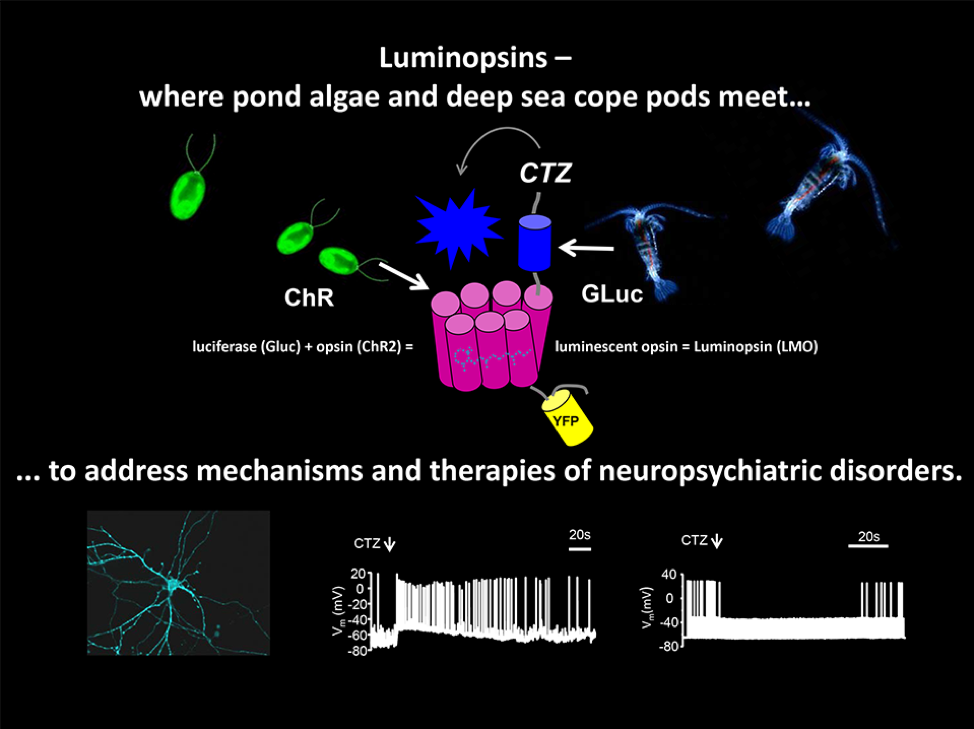
Dr. Ute Hochgeschwender is using the power of bioluminescence, or light that is produced by living organisms such as fireflies, to activate brain cells. Growing up in Germany where she received her medical degree, she nurtured a healthy curiosity for all aspects of the natural world and how they fit together. Her dual interests in both medicine and philosophy nourished a fascination with the workings of the brain. She still loves learning about developments in different branches of science, such as when she attends the annual BRAIN Initiative Investigators Meeting. “It’s fascinating to see people from different areas,” she says. “You get a mental boost from BRAIN.”
Lately, Hochgeschwender has been developing methods to use bioluminescence to modulate the activity of neurons. Her work stems from optogenetics, an earlier technique which combines fiber optics and genetic engineering to create neurons that can be activated or inactivated by light. By expressing opsins, light-sensing proteins which generate an electrical impulse when they absorb a photon, in neurons in the brain, these brain cells can be controlled by shining light on them.
Neuron control with fiber optics presents a few challenges. First, light can only affect a tiny area of cells; second, implantable devices are not practical within the brain. As a professor of neuroscience at Central Michigan University (CMU), Hochgeschwender is working to overcome these obstacles by creating a system wherein neurons generate their own light. With the resources that her BRAIN awards have provided, she has developed neurons which also express luciferase, a bioluminescent protein which emits light when it reacts with the chemical luciferin.
Hochgeschwender has also been modifying the opsin and luciferase proteins to achieve higher levels of light output and greater activity control. Potentially, the future could see a vast range of applications of light-controlled neurons, each adapted to a specific purpose. “It’s an untapped resource,” she says. “There are many different bioluminescence systems in the natural world.”
Diane Lipscombe

Dr. Diane Lipscombe spends a lot of time travelling around the globe, meeting different scientists and medical doctors to share ideas and findings. Along with Dr. Hochgeschwender and other neuroscientists, she is leading a new multi-center collaboration called the “NeuroNex Technology Hub,” which partners Brown University with CMU and the Scintillon Institute. This collaborative effort is developing and refining bioluminescent tools for neuroscience research.
While a graduate student in London, Lipscombe witnessed a then-new method to visualize ions moving through channels. “It was so cool to see a single protein, watching it open and close,” she says. Hooked on this phenomenon, Lipscombe became a professor of neuroscience at Brown and has made a career investigating voltage-gated calcium ion channels. Dysregulation of these channels can result in neurological problems, such as psychiatric disorders, epilepsy, or chronic pain. Understanding the cellular mechanisms of calcium channel control will open the way for treating these and other neurological conditions.
With Hochgeschwender as a partner, Lipscombe has been developing techniques for using bioluminescence to observe calcium channel activation in neurons. BRAIN resources have provided access to technology and expert assistance to create a system for calcium-stimulated luciferase activity. “It really helped us; it allowed us to try potentially risky new ideas,” she says. The work from this initial partnership led to the $9 million award from the NSF to fund their NeuroNex project.
For the time being, Lipscombe’s neurons that glow in response to calcium signaling are especially helpful for visualizing neural circuitry when conducting research. In the future, perhaps, controlling neuron activity by intrinsic light emission may be an effective means to restore function to damaged or diseased cells.